当前位置:首页 > 行业资料 > 国内外标准规范 > 2.Covalent-Bonding
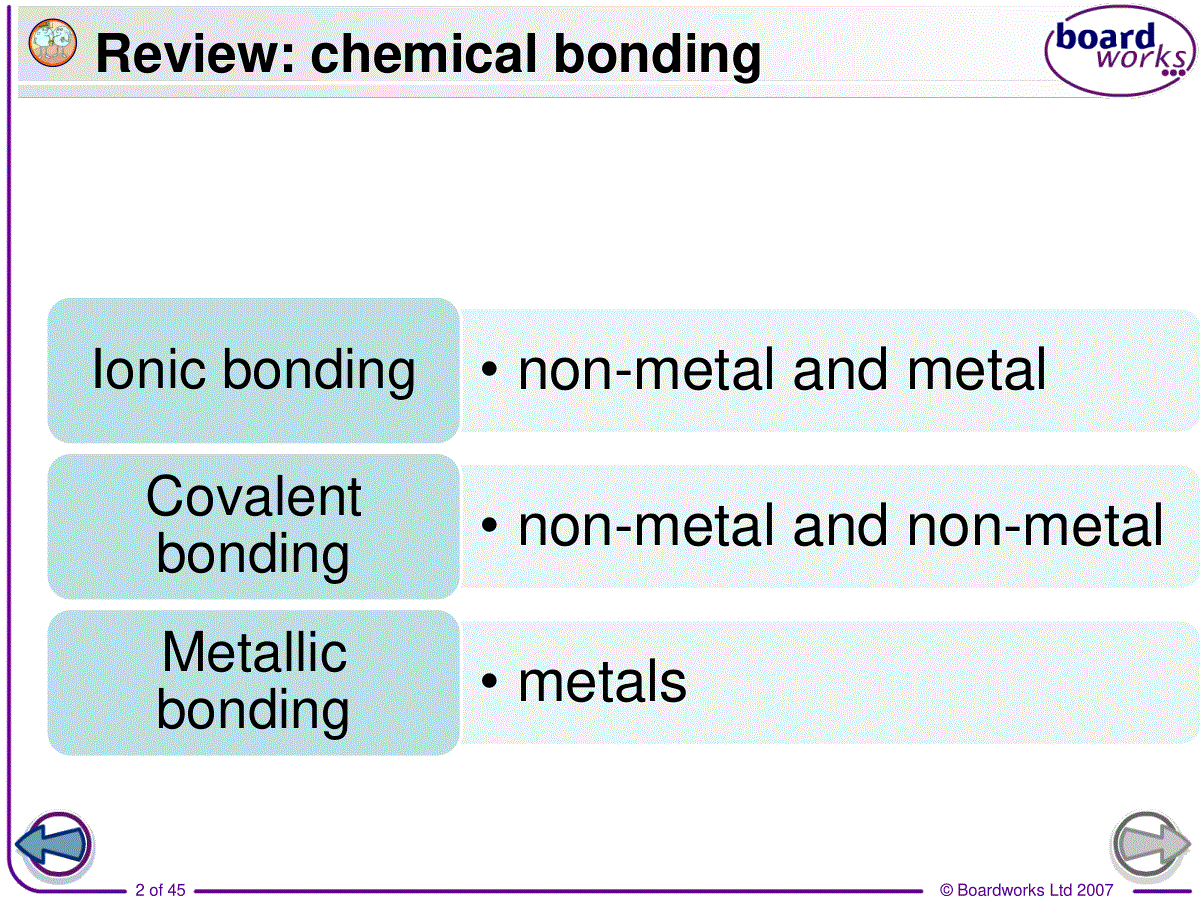
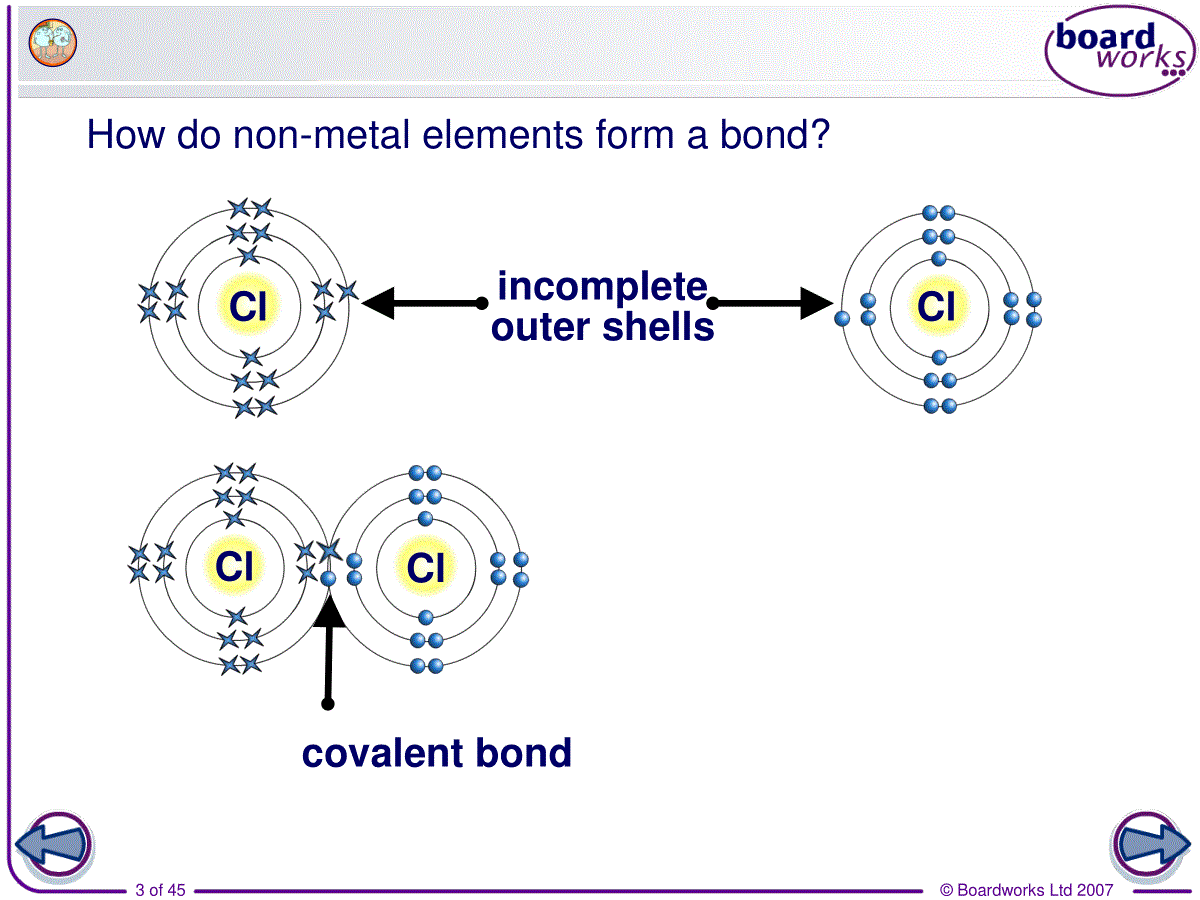
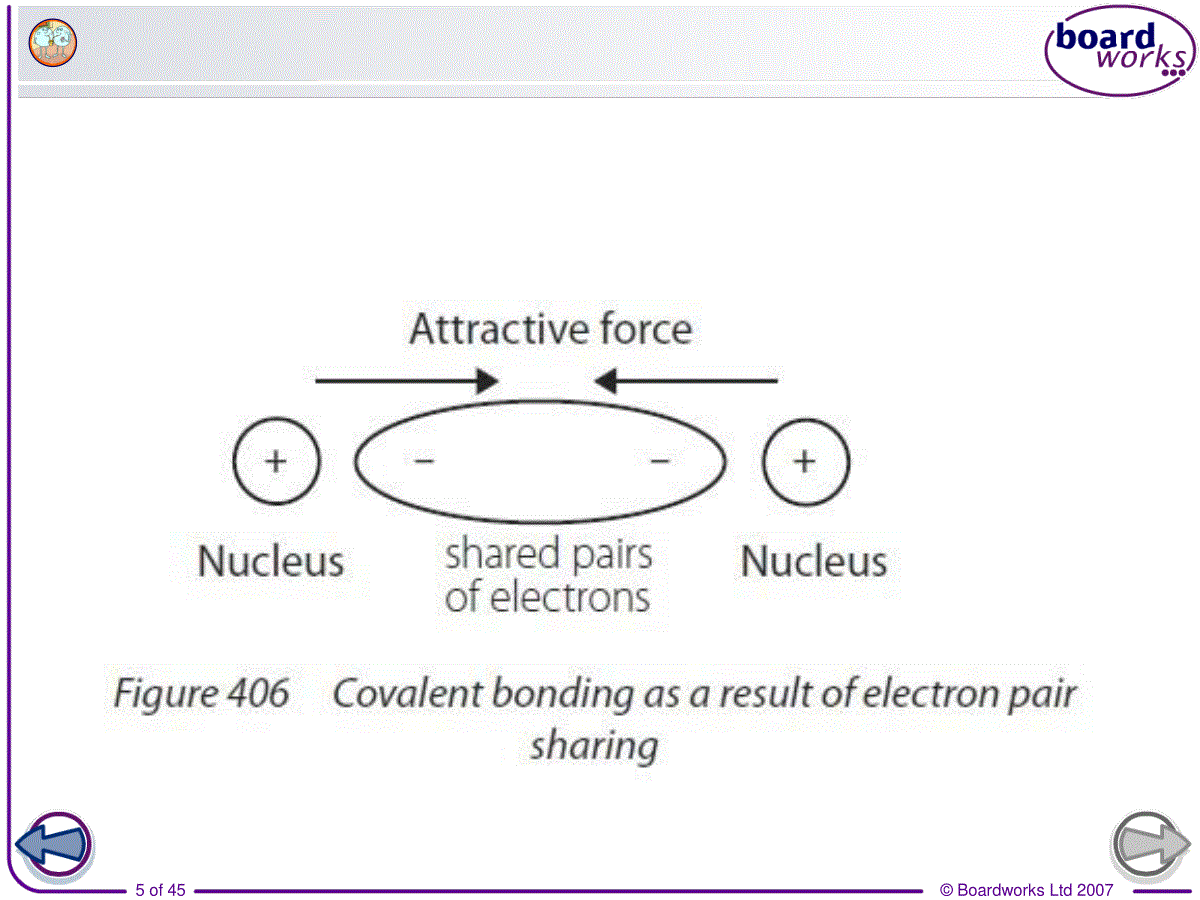
©BoardworksLtd20071of453.covalentbonding共价键©BoardworksLtd20072of45Review:chemicalbonding•non-metalandmetalIonicbonding•non-metalandnon-metalCovalentbonding•metalsMetallicbonding©BoardworksLtd20073of45Howdonon-metalelementsformabond?incompleteoutershellsClClClClcovalentbond©BoardworksLtd20074of45Covalentbonding•(1)Definition:•Achemicalbondformedbythesharingofoneormorepairsofelectronsbetweentwoatoms.•Theattractionofthe2positivelychargednucleusandthesharedpairsofelectronsiscalledcovalentbond.©BoardworksLtd20075of45©BoardworksLtd20076of45•Covalentbondsisusuallybetweentwoormorenon-metalsinthemoleculesorangiantcovalentstructures.Whydoesnon-metalsalwaysformcovalentbonding?•Covalentbondingoccursbetweenelementsthathavehighelectronegativity.©BoardworksLtd20077of45Review:electronegativity•Relativeabilityofanatomtoattractapairofelectronsinacovalentbond.©BoardworksLtd20078of45Comparingcovalentandionicbonding©BoardworksLtd20079of45(2)Octetrule八电子规则•1)Atomsincompounds(exceptforH)usuallyhave8electronsintheirvalenceshell.[moststable]•Atomswill-lose/gainelectrons(ionicbonding)-shareelectrons(covalentbonding)tomakeafilledoremptyouterlayer.©BoardworksLtd200710of45•2)Thenumberofbondsformedisequaltothenumberofelectronsneededforfullvalencelevel.©BoardworksLtd200711of45(3)typesofcovalentbonds©BoardworksLtd200712of45•1)singlebond:Acovalentbondinwhicheachatomsharesoneofitselectrons.e.g.F2,Cl2,Br2,I2,HCl,CH4,CF4,CCl4,H2,C2H6(ethane)•2)doublebond:Acovalentbondinwhicheachatomsharestwoofitselectrons.e.g.O2,CO2,C2H4(ethene)©BoardworksLtd200713of45•3)triplebond:Acovalentbondinwhicheachatomsharesthreeofitselectrons.e.g.N2,C2H2(ethyne)•4)dativebond(配位键)※:oneoftheatomssuppliesbothelectronsofthesharedpair.e.g.NH4+,H3O+©BoardworksLtd200714of45Exercise:©BoardworksLtd200715of45(4)howtopresentcovalentbonding•1)structureformula结构式Eachcovalentbondisshownasalinejoiningtheatomsinvolved.ClClOONN©BoardworksLtd200716of45•2)Lewisformula路易斯电子式•Showsallthevalenceelectronsinvolved.•Eachelectronpairisrepresentedbyaline/apairofcrosses/apairofdots©BoardworksLtd200717of45StructureformulaLewisformula©BoardworksLtd200718of45StructureformulaLewisformula©BoardworksLtd200719of45StructureformulaLewisformulaLoneelectronpair(non-bondedelectrons)©BoardworksLtd200720of45StructureformulaLewisformula©BoardworksLtd200721of45ExerciseWritethestructureformulaandLewisformulaofthefollowingsubstances.CH4,I2,O2,HCN,H3O+©BoardworksLtd200722of45ThedifferencebetweenC2H6,C2H4andC2H2•C2H6ethanesinglebondbetweenthe2C•C2H4ethenedoublebondbetweenthe2C•C2H2ethynetriplebondbetweenthe2C•Thestrength:triple>double>single•Thelength:triple<double<single©BoardworksLtd200723of45(5)VSEPRtheory价层电子对互斥理论valenceshellelectronpairrepulsion•1)Definition:•Thepairsofelectronsarrangethemselvesaroundthecentralatomsothattheyareasfarapartfromeachotheraspossible.•Repulsionbetweennon-bondedpairsofelectrons>repulsionbetweenbondedpairs©BoardworksLtd200724of45•Onepairofnon-bondedelectronsarecountasonenegativechargecenter.•Singlebondsarecountasonenegativechargecenter.•Onedoubleortriplebondiscountasonenegativechargecenter,too.©BoardworksLtd200725of45©BoardworksLtd200726of452)divisiona)2negativechargecentersexamplesShapeandbondangleCarbondioxideOCOLinear180°BerylliumchlorideCl—Be—ClLinear180°©BoardworksLtd200727of45•b)3negativechargecentersexamplesShapeandbondangleBorontrifluorideTrigonalplanar(平面三角形)120°SulfurdioxideV-shaped117°©BoardworksLtd200728of45C)4negativechargecenters正四面体三角锥折线型/V型©BoardworksLtd200729of45•WhythebondangleofNH3issmallerthanthatofCH4?•Greaterrepulsionbynon-bondingpair•WhythebondangleofH2Oisevensmaller?•Evengreaterrepulsionbytwonon-bondingpairssobondangleevensmaller©BoardworksLtd200730of45d)5negativechargecenters※正六面体锯架型©BoardworksLtd200731of45Whataretheallotropesofcarbon?Diamondandgraphiteappeartobeverydifferentsubstancesbutwhatdotheyhaveincommon?Bothdiamondandgraphitearemadeupofcarbonatoms.Theseallotropesofcarbonhavedifferentpropertiesbecausetheatomsarebondedindifferentarrangementswhichcreatedifferentgiantstructures.Differentformsofthesameelementarecalledallotropes.©BoardworksLtd200732of45Whatisthestructureofdiamond?©BoardworksLtd200733of45Whatisthestructureofgraphite?©BoardworksLtd200734of45Bondingandstructure©BoardworksLtd200735of45Multiple-choicequiz©BoardworksLtd200736of45Homework•P59/1(structureformula&lewiscovalentformula),2•P67/3


 三七文档所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
三七文档所有资源均是用户自行上传分享,仅供网友学习交流,未经上传用户书面授权,请勿作他用。
本文标题:2.Covalent-Bonding
链接地址:https://www.777doc.com/doc-7491782 .html